Quantum mechanics is probably one of the most interesting yet confusing branches of science
Here’s a complete guide to making people understand the basics of quantum mechanics, all in very simple terms
Summary of this guide-
Introduction-very basic point by point introduction to quantum mechanics for beginners
What are the laws of quantum mechanics-5 most basic laws of quantum mechanics i.e. superposition, tunneling, entanglement, uncertainty principle, and energy quantization explained with examples
How quantum mechanics developed-how were the basic laws of quantum mechanics developed, along with the experiments which led to their discovery
Quantum mechanics equations-basic mathematical equations explained in simple English
Quantum mechanics interesting facts- 8 most interesting quantum mechanics facts, listed and explained
Quantum mechanics in everyday life-10 interesting applications, along with a simple explanation for their working
Where quantum mechanics fails-quantum mechanics fails to explain macroscopic phenomenon
Introduction to quantum mechanics
Quantum mechanics is a study involving particles at atomic and subatomic levels such as electrons
1. Quantum mechanics is different from classical mechanics, which describes the behavior of macroscopic particles or in simple terms the particles we can see through our eyes.
2. The laws of quantum mechanics are different from classical mechanics, making it hard to visualize and understand.
3. In our normal physical world, we take the position and state of an object to be well defined and independent of other objects, which is exactly opposite to the laws of quantum mechanics
4. Quantum particles do not have a specific position or state, but they are described mathematically, using a wave-function (a mathematical representation of a wave)
5. The wave-function of a particle can give us the probability of finding a particle at a point
6. When we find the particle at a point, the wave-function collapses at that point
7. The barrier between the quantum world and the physical world is a measurement, the moment we make a measurement wave-function shifts to a particle
8. The the gap in our knowledge is that we do not know how a widely spread wave collapse into a localized particle, after we make a measurement at a point
What are the laws of quantum mechanics-
The counterintuitive nature of quantum mechanics lies in the most fundamental laws of quantum mechanics, the fundamental laws are-
1-Superposition
2-Tunneling
3-Entanglement
4-Heisenberg uncertainty principle
5-Energy quantization
1 superposition principle-
The superposition principle starts that a particle can be in it's all possible states all at once and at the same time
Mathematically, a particles wave-function is made of superposition (or addition) of many other waves, where each wave describes a state of electron
1. We can also find the probability of finding a particle from its wave-function at a particular point
2. As the wave-function is a combination of various other waves for different states, it can be said that there are some probability of finding a particle in every possible stat
3. hence the particle is existing in all possible states at the same time
Example-
We consider an electron inside an atom
According to classical mechanics, we ma visualize electron as a kind of object, revolving around a specific point
But, according to quantum superposition, the electron is not revolving or moving from one point in its circular path to others, but it is already present at each and every point in its path
According to this description, we can conceder electron as a the cloud around the nucleus, the dancer the cloud, the more the probability of finding an electron at that point
As we make a measurement, this cloud fades away leaving an electron (particle) behind
An electron is-
1- classical visualization- a particle revolving in an orbit
2- quantum mechanical visualization- a cloud around the nucleus, the density of cloud describes the probability of finding the an electron at that point
3- after measurement- this cloud fades away, leaving the electron(particle) behind
Note- the electron cloud is not physical, a pictorial representation of the wave function of the electron, also known as orbital
Electron is actually present in an orbital, not in an orbit.
2 quantum tunneling-
Quantum tunneling is a principle in quantum mechanics, allowing quantum particles to cross a barrier, by going through the barrier, or tunneling through the barrier
This can be visualized, by a macroscopic example of a ball (particle) and a wall (barrier)
According to classical physics, if we throw the ball toward the wall, it will bounce back
But quantum mechanics tells us, that ball can even be found on the other side of the wall
1- classical mechanics- the ball bounces off the wall
2- quantum mechanics- there is a possibility of the ball is found on the other side of the wall
NOTE- it should be noted that quantum mechanics says it is POSSIBLE to find the ball on the other side of the wall, it doesn’t guarantee that ball will be on the other side.
Mathematically, we can describe quantum tunneling using the the wave function of the particle
1-
before the barrier, the wave-function is having
a high amplitude, so there are good chances of finding the particle before the
barrier
2-
inside the barrier- the wave-function decreases
until the barrier ends, so the chance of finding the particle at the beginning
of the barrier are higher than where the barrier ends
3-
after the barrier- the wave function now remains
at its decreased value, meaning there is a small chance of finding the particle
at the other side of the barrier
NOTE- it must be noted that there is a POSSIBILITY for particle being found even inside the barrier
Example- quantum tunneling naturally takes place inside the sun and nuclear power plants
1- protons are positively charged particles, so they repel each other(like charges repel)
2- inside the sun/nuclear plant, occasionally these protons can tunnel into each other
3- the barrier here is the force that repels protons, protons tunnel through this barrier
4- this combining of protons converts hydrogen into helium, releasing energy
5- this is where the sun’s energy and nuclear power plant energy comes from
3 quantum entanglement-
The phenomenon of quantum entanglement allows one particle to affect the state of another entangled particle, depending on how the first particle is measured
Quantum entanglement works regardless of the distance between the entangled particles
Suppose there are 2 entangled particles, and we measure their spins (or angular momentum)
1- if we measured the first particle’s spin to be spin up
2- the second particle’s spic will certainly be spin down
3- if the first particle’s spin was spin down
4- the second particle’s spin will be certainly spin up
here we can see that it doesn’t depend what was the spin of first particle, the spin of the second particle will always be opposite to the first
It seems that there is a communication taking place between the particles, as if the first particle is telling the second particle, what your spin should be
Entanglement works even if particles are astronomically far away, this seemed there is a faster than light communication between them (not allowed in Einstein’s theory of relativity)
Einstein didn’t like entanglement, so he called it ‘spooky action at a distance
Mathematically we can explain quantum entanglement, and say it is not a faster than light communication.
1- Consider 2 particles with different wave functions(defining their respective states)
2- These particles interact with each other
3- After interaction, there is only 1 wave-function for both
4- The states of the particles will be defined by this single wave-function
5- Measurement of the state of the first particle using this wave-function changes the wave-function (defining the state of the second particle), so the state of the second particle is affected
Inlayman’s terms, we can simplify it as-
1- Y= -X is a wave-function, defining spins, for 2 entangled particles X and Y
2- if the spin of particle X is spin up or X=1
3- spin of Y is, Y= -(1) = -1 or spin down
4- if X= -1 (spin down), then Y= -(-1)= 1 (spin-up)
This is how entanglement works
NOTE- this is just an example to make you feel how quantum entanglement works
Example-in 2018 scientists found entanglement between a green sulfur bacteria and quantized light; this was for the first time entanglement was found in a living creature.
The electrons in electron shells of an atom are always entangled
4 Hinesburg’s uncertainty principle-
Hinesburg’s uncertainty principle says that you cannot know or measure the position and momentum of a particle accurately at the same time
This principle is not because of flaws in measuring instruments, but it is a fundamental limit to measurement
1- wave-function of a particle contains all information about its position and momentum
2- the amplitude squared tells the position and wavelength tells the momentum
3- if we fix the wavelength, we get a sine wave
4- we square this wave to find the probability distribution for particle’s position
5- we get a wave with constant amplitude, stretching to infinity on both sides
6- meaning that particle can be found anywhere in this universe or we are completely uncertain about the particle’s position
Example-suppose we want to find the position of an electron
1- we can only see an object which eater emits or reflects light
2- for an electron to reflect light, it must collide with particles of light (photons) and reflect them back
3- as soon as electron collides with light particles, it’s momentum (or velocity) changes
4- now we know the position of an electron, but don’t know it’s momentum
NOTE-it may seem that this is a limitation of the instrument but, instead, it’s a fundamental limitation of measurement, regardless of instrument.
5 Energy quantization-
Quantization of energy means that energy cannot randomly have any value, but certain discrete, allowable values.
Energy quantization was used to explain, why lights of only certain wavelengths are present in the spectrum of atoms.
1- Suppose an atom is excited with certain energy
2- Electrons of an excited atom moves to higher energy levels
3- When these electrons come back to their original energy levels, they emit lights
4- These lights are of certain wavelengths only
5- This indicates that only a fixed amount of energy is absorbed and emitted by atoms
6- Thus, energy in an atom’s energy level only has certain discrete values.
Another example of energy quantization is found in light
1- Light have small packets of energy called photons
2- These packets only have a definite value of energy
3- Some of the energy in all the photons present gives the total energy of light.
How quantum mechanics developed-
Quantum mechanics was developed, when classical mechanics couldn’t explain certain physically observed and experimentally verified phenomenons.
Here’s how these 5 basic quantum mechanical laws were developed
1- Energy quantization- by studying radiations emitted from a hot object
2- Superposition- young’s double-slit experiment
3- Entanglement- mathematically
4- Tunneling- experiment to study Andreev reflection
Discovery of energy quantization (beginning of quantum mechanics)-
The development of quantum mechanics was a consequence of a series of phenomenon’s and problems which were not at all explainable with the classical knowledge of the world, hence certain mathematically deduced counterintuitive laws begin to enter science and they collectively formed the the base for quantum mechanics
One such problem was, the problem of increasing the power of a light bulb
1- Taking classical or wave nature into account, for making more powerful light bulbs, we need to have more light as visible light, emitted from the bulb
2- The bulb emits light from a heated filament
3- If we plot a graph between the temperature and the light emitted from an object, the plot doesn’t match with experimental evidence
4- Considering energy emitted from lights in the form of small packets of light, with fixed energy, perfectly explains experimental observations
This is how the idea of treating light as a collection of a very large number of energy packets, for each wavelength in light, gave rise to energy quantization.
NOTE-it must be noted that, energy in the energy packet of a particular the wavelength, is fixed, meaning it won’t give or take any energy different from that discreetly defined energy.
Quantum superposition and the further development-
Quantum superposition was used as an explanation, for the famous, young’s double-slit experiment
1- When an electron beam is passed through 2 slits, they form a striped pattern behind them, with several alternate light and dark strips
2- Considering electron as a particle, there would have been only 2 light strips, right behind the slits.
3- Here particle nature of electron doesn’t explains the observation
4- But, when an electron beam is considered as waves, the pattern observed is completely explained
This showed electrons also had wave-like properties, but these waves collapsed upon measurement, to become a localized particle, from here came the concept of quantum superposition
Problems in the model of an atom and the uncertainty principle-
The uncertainty principle was discovered from the very nature of matrix multiplication that AxB is not always BxA
The model of the atom was thought of as an electron revolving around the nucleus, this atom worked fine for hydrogen, but not for multielectron atom systems.
1- Heisenberg developed a set of matrices to describe the atomic phenomenon
2- These matrices worked fine and explain all the phenomenon
3- From the very nature of matrix multiplication, the uncertainty principle was born
4- It was later also confirmed that uncertainty the principle was a fundamental limitation, and not the fault of the instrument used
NOTE-it must be noted that the uncertainty principle not only applies to position and momentum, but also to energy and time, spin on different axes etc.
The accidental discovery of quantum tunneling-
Quantum tunneling was discovered in an experiment, which was not even designed to study it, but is accidentally discovered tunneling
Here’s the experiment
1- When a metal and superconductors are brought close, electrons from metal jump into a superconductor, this called Andreev reflection
2- If electrons from metal successfully jumped to superconductor, the conductivity of superconductor should double
3- Doubling of conductivity is difficult as all electrons don’t have the required energy for the jump (energy barrier)
4- This experiment was done with samarium and hexaboride
5- The conductance was found to be perfectly double, even after repeating this experiment multiple times
6- Quantum tunneling was suggested as a possible explanation (electrons tunneled through the energy barrier)
The EPR paradox and quantum entanglement-
The concept of quantum entanglement was developed solemnly using math and equations of quantum mechanics
Later, Einstein with 2 other scientists conducted a thought the experiment is known as EPR paradox
1- 2 entangled particles are separated by a large distance
2- The spin of the first particle is measure along an axis
3- According to spin conservation the spin of the second particle should be exactly opposite to the first one
4- This works, even when the spin of the first particle is measured differently, the total spin is always conserved
5- It seems here that particles are somehow, able to instantly contact each other, as soon as anyone of them is measured, this is the paradox
Einstein along with other scientists concluded that quantum mechanics was incomplete, as they were not able to explain how particles communicated
Quantum mechanics equations-
Here are the quantum mechanics equations for the basic laws of quantum mechanics
1) Energy quantization; E = nhν
2) Uncertainty principle ΔX*ΔP = (h/4π)
The other laws can be seen in mathematical form when we use the wave function for the particle waves
1- The wave function of a particle is a mathematical equation, which describes the position, spin, and various other properties for the particle denoted by ψ
2- In classically this task is done by Newton’s explanation
3- Except, in classical mechanics the particle has a fixed location before and after measurement
4- In quantum mechanics, a particle’s location is not perfectly known, we can only tell the probability of its presence at a location
5- This probability is known from the square of the wave function’s amplitude i.e. amp(ψ)^2
3) Quantum entanglement and superposition- bells states
4) Quantum tunneling- it is described by 3 equations, 2 for both sides of the barrier and 1 for inside the barrier
A)
A![]() + B
+ B![]()
B)
K![]()
C)
C![]() + D
+ D![]()
Explaining these equations in simple English
1) The equation E =nhν is one of the very first equations in the mathematical quantum realm
Here,
a)
E is the total energy emitted by a light source
b)
n is the total number of energy packets
emitted (photons)
c)
ν is the frequency of light
This equation tells that energy has certain discrete values only,
because ‘n’ is always a natural number
2)
The mathematical form of the uncertainty principle
is exactly what it says
Here,
a)
ΔX is uncertainty or change in
position, if we are unsure about the exact position, but know a range of position,
this range is denoted by ΔX
b)
ΔP is uncertainty or change in
momentum, similarly the range of possible momenta is ΔP
c)
(h/4π) is simply a constant number
When we know one of X or P certainly, then ΔX or ΔP become 0 and the corresponding value of change in other value becomes infinite
Example-
1- if we know the position for sure then ΔX = 0
2- from the equation ΔP = infinite
3- meaning we are completely unsure about momentum, it can be anything from negative infinity to positive infinity
3) Quantum superposition is seen mathematically as various possible states in the wave-function of a particle
All these states are usually separated by + signs in the wave-function
Example-
1- before measurement the wave-function of an electron, describing its spin is ψ = A + B
2-
here, A = electron spin is ![]() , and B = electron spin is
, and B = electron spin is ![]()
3- thus this wave-function indicates that electron has both the spins, before measurement
4-
after measurement, if spin comes out to be ![]() , wave-function collapses to ψ = A
, wave-function collapses to ψ = A
5-
if the spin was ![]() , wave-function becomes ψ = B
, wave-function becomes ψ = B
This is how superposition is seen mathematically
in a similar way quantum entanglement can also be verified, before understanding, we must keep in mind that the coefficient squared of state in a wave-function is the probability of being in that state
·
if ψ = a(u) + b(d), u = up spin, d = down spin
·
probability of u = ![]() , and the probability of d =
, and the probability of d = ![]()
The seemingly difficult concept of entanglement has a very the simple math behind it; now let’s dive right into the mathematics of quantum entanglement
1- consider a common wave-function ψ for 2 entangled electrons A and B
2-
ψ = ![]() AuBd +
AuBd + ![]() AdBu , here
Au = A up, Bd =
B down etc.
AdBu , here
Au = A up, Bd =
B down etc.
3-
probability of Bd before measuring A is ![]() , so B can be up or down, both have a
50% chance
, so B can be up or down, both have a
50% chance
4- after measuring A as Ad the wave-function becomes ψ = AdBu here the probability of Bd is 0, thus B is certainly spin up
5- if A was measured Au ψ = AuBd , so B is certainly spin down
6- this clearly indicates that the state of B changes, depending on A
NOTE-
the An example of wave-function taken here is known as bell’s states.
4)
The tunneling equations-
a)
A![]() + B
+ B![]()
b)
K![]()
c)
C![]() + D
+ D![]()
Here,
· A, B, C, D, K are constants (numbers)
· X is the distance
·
i=![]()
These equations simply represent, that the probability of finding the particle, outside the barrier varies sinusoidal, whereas inside the the barrier it varies exponentially
Quantum mechanics in chemistry
Quantum mechanics in chemistry, plays a major role in explaining the atomic phenomenon and modeling an atom
Before quantum mechanics various other atomic models were suggested, some of them are
A) plum pudding model- describing atom as a solid sphere with electrons scattered inside it, and positive charge distributed uniformly inside just like a watermelon
B) Rutherford’s model- it describes atom similar to plum pudding model, except, it considers the positive charge to be centrally concentrated, rather than distributed
C) bohr’s model- suggesting that electrons revolve around a central positive charge called the nucleus
All these models have some problems, as they cannot explain some experimentally observed results
The Quantum mechanical model was later suggested as atomic model and it explained all the results.
Here are the basic features of this model, and how is quantum mechanical model different-
1- electrons are treated as waves, called matter waves
2- mathematically electron waves are expressed as a wave-function ψ (superposition)
3-
probability of finding an electron is ![]()
4- atom has orbitals instead of orbits
5- 2 electrons in 1 orbital never have the same spin
6- position and momentum of electrons, cannot be known at once (uncertainty principle)
7- the energy emitted or absorbed by an atom has fixed values (energy quantization)
This model of an atom is, by far, the most accurate model of atom
The orbitals in the quantum mechanical model of an atom, are simply graphs
of the electron wave, they help us know where it is most likely to find the
electron
In simple terms, an orbital is a region in space, having the the highest probability of finding an electron in it
The Quantum mechanical model, talks about probability of finding the electron in a a particular state, because before measurement electron is present in superposition of its all possible states
Quantum mechanics interesting facts
Here is a list of some of the most interesting quantum mechanics facts
1- being present at so many places, at the same time
2- illusion of reality
3- existence of multiverse
4- time does not exist
5- affecting something light-years away
6- traveling through walls
7- empty space is not empty
8- teleportation
9- affecting the past (most interesting)
1- being present at so many places, at the
same time-
This is a direct consequence of the superposition principle
This principle allows objects at the atomic scale to be present in all their possible states, that too at the same time
This way the state of the object is the sum of all the possible state
The condition for being present in a superposition state is that, no one should look at the particle
The moment anyone observes the particle, these states collapse, leaving only one state behind
2- the illusion of reality-
This is also a consequence derived from the superposition principle
The reality which we observe is actually a collapsed state out of many states of a particle
In other words, the reality is an illusion, because before observing particle didn’t exist, as we saw them
Before observation particle were like waves or a superposition.
In other words, the reality we observe is a result of our observation, it is not fundamental
3- the existence of multiverse-
The existence of a multiverse is used as a theory to explain the superposition principle
It is said that whenever an object is in a superposition state, all these states exist separately in different parallel universes
Whenever an observation is made, a series of events happen, which may be equal to the number of states
Example- we place a cat in a box with a bomb(which is equally likely to explode and not explode), and observe it
a) if we find the cat dead, with a bomb busted
b) then the cat is also alive, with a bomb unexploded, but in a parallel universe
c) and vice-versa
Note- multiverse theory may be correct, as recently NASA has supposedly observed a parallel universe moving backward in time
4- time does not exist-
Time may seem to move forward in our macroscopic world, but at the quantum, level events don’t take place in a specific order
The chaotic sequence of events, leads us to conclude that time does not exist at the very basic level of universe
5- affecting something light-years away-
This is a direct consequence of quantum entanglement
2 entangled particle can affect each other, regardless of the distance between them, so it works even if particles are a light-year away
The reason behind entanglement is the common wave-function for both the particles or both the particles are part of the same wave
Entanglement is not only a theoretical concept, but it has been experimentally observed as well
Entanglement can be combined with the superposition principle and, be explained with the multiverse theory
Example- consider a cat with a bomb in a box
a) the moment cat, bomb and anything is placed inside the box, they become entangled, after we open the box to observe the cat, we to get entangled with this system
b) now the bomb and the cat are entangled
c) if the bomb explodes, the cat dies, and we observe a dead cat
d) if the bomb remains unexploded, the cat survives, and we observe a live cat
e) due to entanglement, no other combination is allowed
f) As we open the box, the wave function collapses, and these sequences of events occur but in parallel universes.
Note- this is just a thought experiment
6- traveling through walls-
The phenomenon of quantum tunneling allows the tiny atomic scale particles to be able to travel through walls, or any kind of barrier present in their path
This phenomenon is not only theoretical but has been experimentally observed
Tunneling along with some other quantum phenomenon can be explained with the help of pilot-wave theory
In fact, quantum tunneling can also, be observed at the macroscopic scale using oil droplets.
7- Empty space is not empty-
Even the purest intergalactic space having only 1 or 2 atoms in it, is not that empty, even a space with 0 atoms in between is not empty
The reason for this is virtual particles
Virtual particles are particle pairs which are formed out of nowhere in space, and annihilate back into energy
Usually, virtual particles consist of a particle and its antiparticle
Virtual particles cause a change in the energy of space at a point, this is known as a quantum fluctuation
8- Teleportation-
Teleportation is allowed for quantum particles, but this teleportation is quite different from the one which is seen in sconce fiction
Quantum teleportation allows teleporting the quantum state of a particle, in other words, the information to create the particle
Some scientists even suggest that the teleportation of energy may also be possible, by using an entangled vacuumed state of a quantum field.
Teleportation of information between 2 entangled particles, 3 meters apart, was demonstrated in 2014
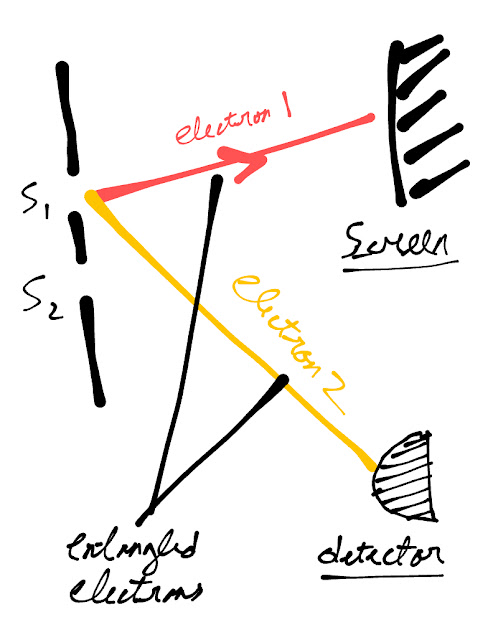
9- Affecting the past-
One of the most fascinating experiments in quantum mechanics is the delayed choice quantum eraser experiment
But before going into details, let’s see how particles behave
· When we observe particles, they become particles, and form only 2 bright strips
· When we don’t observe particles, they become waves
Keeping this behavior of particles in mind lets describe an outline of the delayed choice quantum eraser experiment
Ø The setup of this experiment is a modification of young’s double-slit experiment with some detectors
Ø 2 entangled electrons are emitted from each slit
Ø The 1st entangled electron forms pattern on the screen, while 2nd goes to the detector, the detector tells from which slit both these electrons came
Ø The 2nd electron reaches the detector reaches 8 nanoseconds later, then the 1st electron which reaches the screen
Ø When the 2nd electron is observed, 1st electron shows particle behavior, and form 2 bright stripes
Ø With a quantum eraser in the arrangement, the information, from which slit the electrons came is erased
Ø Adding a quantum eraser is similar to not observing the electrons
Ø Now when the 2nd electron is not observed, the 1st electron shows wave behavior and form consecutive bright and dark lines
Ø It appears that somehow, the 2nd electron tells the 1st electron, what kind of pattern to form
Ø The electron 1st reaches early on screen, so it has to travel back time and form the pattern, based on the 2nd electron is observed or not (which reaches later)
Quantum mechanics in everyday life-
Quantum mechanics, because of its counter-intuitive nature may appear to be useless science fiction, but the truth is completely opposite
Without quantum mechanics, it would even be difficult to imagine this world, quantum mechanics actually changed the world
So here is a list of quantum mechanics uses in our daily lives
1- Fluorescent light (LEDs)
2- LASERs
3- Smartphones and computers
4- Communication using fiber optics
5- GPS system
6- MRI scan
7- Digital camera
8- Scanning tunneling microscopy
9- Birds
1-
Fluorescent lights (LEDs)
Fluorescent lights are based on energy quantization
It would be better to say that energy quantization can be used to determine the color of fluorescent lights
Fluorescent lights emit lights in the very same an atom emits lights when its electron jumps down from an excited state
The amount of energy emitted in a jump from one energy level is fixed
So the corresponding wavelength of light emitted is also fixed
LEDs are extremely power efficient, that’s why they are their usage is increasing day by day
Right from your mobile screen to your room lights, use these quantum lights!
2-
LASERS
LASERS are similar to EDs, except they have a very narrow beam, consisting of almost a single colored light
The production of lasers involves the use of stimulated emission, which is a quantum mechanical phenomenon
This is very similar to the process of light emission in LEDs, except, here the electron is forced to jump from an energy state, but in LED electron jumps on its own
LASERS have made a lot of industrial processes easy and time efficient
They are used in the processes of inveigling cutting a material preciously
3-
Smartphones and computers-
Almost all modern computing relies upon quantum mechanics for its functionality
The understanding of the wave nature of matter and electrons helps to understand and develop semiconductor devices such as transistors
Millions of transistors are used together to make a computer a chip or a microprocessor, which powers the entire generation, right from small music players to supercomputers
Apart from the microprocessor, as described above, quantum mechanics also used in making LEDs which are used behind the screen of every device which has a screen.
(also see: quantum computers explained simply)
4-
Communication using fiber optic-
Fiber optic cable is a special kind of cable which reflect the light inside them in such a way that, if we send light from one end it will be visible on the other end, even after bending the cable
Communication is made through these cables using LASERs, and as explained LASERs are possible only because of quantum mechanics
Thus we can conclude quantum mechanics have an indirect role in fiber optic communication
5-
GPS system-
The technology that helps you know, where you are at the moment, global positioning system is also made possible using quantum mechanics
Broadly there are 2 systems used in this process-
Ø Your mobile phone
Ø GPS satellites
And quantum science is used at both the ends
The microprocessor in your phone and the atomic clocks in satellite use quantum mechanics
Atomic clocks ticking depend on oscillations of microwaves between 2 energy states of the cesium atom
6-
MRI scan-
Magnetic resonance imaging relies on the phenomenon of nuclear magnetic imaging
The spin of the nucleus of the hydrogen atom is flipped and then with an arrangement of magnetic fields, the amount of hydrogen in the body is measured
Hydrogen makes many of the softer tissues visible in MRI scan, which was not possible in an x-ray scan
You might be wondering, where is quantum mechanics in this process?
Quantum mechanics here is in the very basic property of an atom
“It's spin”
7-
Digital Camera-
Digital camera sensors are made up of something called a photodiode
The photodiode is a kind of semiconductor device which releases current when light falls on it, this current is then understood by the camera to make a photograph
Megapixels of cameras are increasing day by day because we can manipulate the energy levels in semiconductor of photodiodes
8-
Scanning tunneling microscopy-
Scanning tunneling microscopy is another interesting use of quantum mechanics, as the name suggests, this technique use quantum tunneling to zoom in
Scanning tunneling microscopes are so powerful, that they can even zoom up to the level of atoms
In a way, we are peeping into the quantum world by using quantum mechanics
The steps in working of scanning tunneling microscopy are-
· a very fine metal wire tip is brought very close to the surface which needs to be scanned
· a voltage is applied between the sample surface and metal tip
· because of tunneling, electrons from the sample surface, tunnel through the distance barrier between sample and metal wire tip
· this causes a current flow in metal wire
· metal wire tip is moved over the surface and respective current density is measured
· applied voltage vs. current density is plotted on a graph
· this data is analyzed to see the surface atoms
9-
Birds-
Birds such as European robin use quantum entanglement to keep a track of their location, when they migrate from one place to other
This is one of the most fascinating uses of quantum mechanics used not by humans, but by birds
Here’s how birds use entanglement
Ø birds have light-sensitive protein cells
Ø these protein cells have entangled electrons
Ø as light particles (photons) enter through bird’s eye, they hit these protein cells
Ø this collision between protein and light splits protein and entangled electrons in 2 parts
Ø but the electrons are still entangled (entanglement doesn’t depend on the separation between particles)
Ø magnetic fields in bird’s environment determine how protein splits
Ø bird’s eyes are thought to be very sensitive to these parts of a protein
Ø so birds can figure out the magnetic field outside them, this helps in keeping a track of their location
Where quantum mechanics fails-
Quantum mechanics fails in describing macroscopic systems
In other words, objects which are big or are made up of a number of atoms or the things we can see with eyes
We never encounter a weird quantum world phenomenon in our daily lives, such being present at 2 places at the same time, affecting other person, somewhere far away
The main reason behind quantum mechanics failing to explain macroscopic systems are quantum decoherence
Quantum decoherence is a field undergoing research since 1980
Conclusion-
We have covered almost all the basic concepts of quantum mechanics in detail along with relatable examples
Now it’s your turn to tell me, which part you liked the most
Or
If you have any doubt regarding any concept explained
Go ahead and comment!

























Comments
Post a Comment